Abstract
Background: The outcomes in pts with newly diagnosed (ND) FLT3 mutated (m) AML ineligible for intensive chemotherapy (IC) and R/R FLT3 AML are poor. Quiz, a potent FLT3i, demonstrated synergy with VEN in AML cell lines and PDX models (Mali Haematologica 2020). We evaluated the Quiz, VEN, and DAC triple combination in pts with R/R or ND FLT3m AML.
Methods: The ND cohort included pts ineligible for IC, and R/R cohort included pts with ≤ 5 prior treatments for AML (i.e. up to salvage 5). Pts had ECOG PS ≤2, adequate organ function, and QTcF <450 msec at enrollment. All pts underwent Day 14 bone marrow (BM), and VEN (400 mg/day) was held on Day14 in pts with BM blasts ≤ 5% or marrow aplasia/insufficiency. Pts with Day14 BM blast >5% continued VEN for 21 days during cycle 1 (figure 1). Responses were per modified CRc criteria as used in the ADMIRAL and QUANTUM-R registration studies.
Results: 31 pts were enrolled including 25 R/R and 6 ND (Table 1). Of 25 pts with R/R AML (median 3 [range 1-5] prior therapies), 80% had ≥1 prior FLT3i's (72% had prior gilteritinib), and 40% had prior ASCT. In this high-risk R/R Cohort, 2 pts were early to evaluate (still in the first cycle). CRc rate among remaining 23 pts was 65% (15/23) (including CR 13% and CRi 52%).MFC (sensitivity 10 -4) and FLT3-PCR (sensitivity 10 -2-10 -3) negativity rates among responders were 36% (5/14), and 42% (5/12), respectively. The median (med) number of cycles to response was 1 [range 1-2]. 30- and 60-day mortality rates were 4% (N=1) and 16% (N=4). With a med follow-up (f/u) of 7.1 months, the med OS was encouraging at 7.5 months and 1-year OS 34% in this predominantly gilteritinib (gilt) exposed, heavily pretreated R/R cohort. Although numbers were small, pts who received no prior gilt (n=7) had longer OS than pts who received prior gilt (n=18); 19.0 months vs. 7.1 months.
In the ND cohort, 1 pt was not evaluable (switched therapy on Day 2 of Cycle 1). Of 5 evaluable ND AML, all achieved CRc (2 CR, 3 CRi) with 2/4 and 4/5 responders negative by FLT3-PCR and MFC, respectively. With a med f/u of 7 months the median OS was 14.5 months.
No pts developed a dose-limiting toxicity (DLT) with 30 mg/day Quiz, however with the 40mg/day Quiz, the first 2 pts treated developed hematologic DLT (grade ≥3 neutropenia with a <5% cellular bone marrow with no residual AML lasting ≥42 days). Hence, Quiz 30 mg/day dose was declared RP2D. Grade ≥3 non-hematologic toxicities regardless of attribution in >5% included neutropenic fever (32%), pneumonia (32%), sepsis (11%), other infections (7%). No QTcF prolongations >480 msec were noted.
3/5 (60%) and 7/19 (37%) pts underwent ASCT in frontline and R/R cohorts, respectively. Of the 5 frontline pts at the last follow-up; 4 in CR and 1 died with relapsed AML at 14 months. Of 15 CRc pts in the R/R cohort, 8 are alive (7 CR, 1 relapse), and 7 died (6 relapsed, 1 died in CR).
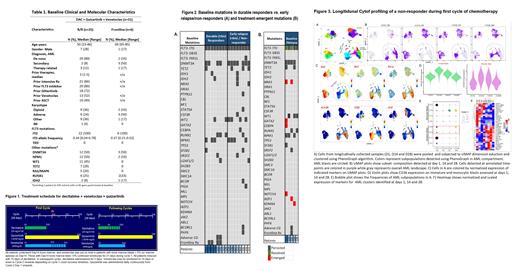
Baseline mutations in durable responders (>6m) vs. early relapse (<6m) and NRs are shown in Fig 2A. Interestingly all 3 pts harboring pretherapy RAS/MAPK mutations were either NRs (n=1) or relapsed early (<6 months) (n=2). Of 5 pts who achieved a response then relapsed with available pre- and post-NGS data, 3 had additional emergent mutations at relapse (Figure 2B.) The most common emergent mutation was NRAS (in 2 of 3 relapses).. 2 of 5 pts lost FLT3 at relapse. We performed CyTOF analysis of longitudinally collected samples to map dynamic therapy response and gain insights into potential mechanisms of resistance. Phenotypic profiling of AML compartment revealed immature AML cells demonstrated that immature AML cells had high Bcl-2 and Bcl-xL and expressed moderate levels of Mcl1 (Figure 3). As expected mature monocytic cells lacked Bcl2 expression and had high Mcl1 levels. We observed that triplet significantly reduced the number of leukemia cells at day 14. Importantly, surviving leukemia and monocytic cells had higher levels of CD36 compared to pretreatment sample, suggesting that fatty acid metabolism may play a role in mediating therapy resistance to triplet therapy.
Conclusion: DAC + VEN + Quiz is active in heavily pretreated and prior FLT3i exposed (including 68% with prior gilteritinib) R/R FLT3-ITDm pts, with a CRc rate of 65% and a med OS of 7.5 months, 1-year OS 34%. Quiz 30mg QDay was established as the RP2D for the triplet. RAS/MAPK mutations continue to be associated with primary and secondary resistance even with this triplet. Accrual continues and updated clinical, NGS and mass cytometry data will be presented.
Yilmaz: Daiichi-Sankyo: Research Funding; Pfizer: Research Funding. Kantarjian: BMS: Research Funding; Daiichi-Sankyo: Research Funding; Precision Biosciences: Honoraria; Astellas Health: Honoraria; Novartis: Honoraria, Research Funding; NOVA Research: Honoraria; Ascentage: Research Funding; Taiho Pharmaceutical Canada: Honoraria; Immunogen: Research Funding; KAHR Medical Ltd: Honoraria; Pfizer: Honoraria, Research Funding; Jazz: Research Funding; Ipsen Pharmaceuticals: Honoraria; Aptitude Health: Honoraria; AbbVie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Astra Zeneca: Honoraria. DiNardo: Foghorn: Honoraria, Research Funding; Novartis: Honoraria; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Forma: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; ImmuneOnc: Honoraria, Research Funding; Agios/Servier: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding. Kadia: Genentech: Consultancy, Other: Grant/research support; Ascentage: Other; AstraZeneca: Other; Pfizer: Consultancy, Other; Pulmotech: Other; Cure: Speakers Bureau; Novartis: Consultancy; Liberum: Consultancy; Dalichi Sankyo: Consultancy; Sanofi-Aventis: Consultancy; Cellonkos: Other; Jazz: Consultancy; Amgen: Other: Grant/research support; Aglos: Consultancy; Genfleet: Other; Astellas: Other; BMS: Other: Grant/research support; AbbVie: Consultancy, Other: Grant/research support. Konopleva: Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Calithera: Other: grant support, Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding; Stemline Therapeutics: Research Funding; Ablynx: Other: grant support, Research Funding; Cellectis: Other: grant support; Forty Seven: Other: grant support, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Agios: Other: grant support, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; AstraZeneca: Other: grant support, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; Sanofi: Other: grant support, Research Funding; KisoJi: Research Funding; Ascentage: Other: grant support, Research Funding. Borthakur: University of Texas MD Anderson Cancer Center: Current Employment; Protagonist: Consultancy; Takeda: Membership on an entity's Board of Directors or advisory committees; ArgenX: Membership on an entity's Board of Directors or advisory committees; Ryvu: Research Funding; GSK: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astex: Research Funding. Pemmaraju: ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; LFB Biotechnologies: Consultancy; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Samus: Other, Research Funding; Plexxicon: Other, Research Funding; Aptitude Health: Consultancy; Springer Science + Business Media: Other; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Consultancy; Blueprint Medicines: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Clearview Healthcare Partners: Consultancy; MustangBio: Consultancy, Other; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Daiichi Sankyo, Inc.: Other, Research Funding; Cellectis S.A. ADR: Other, Research Funding; Bristol-Myers Squibb Co.: Consultancy; Affymetrix: Consultancy, Research Funding; CareDx, Inc.: Consultancy; Roche Diagnostics: Consultancy; Sager Strong Foundation: Other; DAVA Oncology: Consultancy; Incyte: Consultancy; Protagonist Therapeutics, Inc.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy. Short: Jazz Pharmaceuticals: Consultancy; Novartis: Honoraria; NGMBio: Consultancy; Astellas: Research Funding; AstraZeneca: Consultancy; Takeda Oncology: Consultancy, Research Funding; Amgen: Consultancy, Honoraria. Alvarado: FibroGen: Research Funding; Jazz Pharmaceuticals: Research Funding; BerGenBio: Research Funding; MEI Pharma: Research Funding; Astex Pharmaceuticals: Research Funding; Daiichi-Sankyo: Research Funding; CytomX Therapeutics: Consultancy; Sun Pharma: Consultancy, Research Funding. Loghavi: Abbvie: Current equity holder in publicly-traded company; Curio Sciences: Honoraria; Gerson Lehrman Group: Consultancy; Guidepoint: Consultancy; Peerview: Honoraria; Qualworld: Consultancy. Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding. Ravandi: Xencor: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Novartis: Honoraria; Amgen: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Taiho: Honoraria, Research Funding; Prelude: Research Funding; Astex: Honoraria, Research Funding; AstraZeneca: Honoraria; Agios: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding. Andreeff: Novartis, Cancer UK; Leukemia & Lymphoma Society (LLS), German Research Council; NCI-RDCRN (Rare Disease Clin Network), CLL Foundation; Novartis: Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy; Daiichi-Sankyo: Consultancy, Research Funding; Breast Cancer Research Foundation: Research Funding; Karyopharm: Research Funding; ONO Pharmaceuticals: Research Funding; Medicxi: Consultancy; Amgen: Research Funding; Senti-Bio: Consultancy; Glycomimetics: Consultancy; AstraZeneca: Research Funding; Reata, Aptose, Eutropics, SentiBio; Chimerix, Oncolyze: Current holder of individual stocks in a privately-held company; Aptose: Consultancy; Oxford Biomedica UK: Research Funding. Daver: FATE Therapeutics: Research Funding; Sevier: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Trovagene: Consultancy, Research Funding; Hanmi: Research Funding; Novimmune: Research Funding; Glycomimetics: Research Funding; ImmunoGen: Consultancy, Research Funding; Novartis: Consultancy; Genentech: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal